http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html
Sunday, May 8, 2016
Ideal Gas Law
Ideal Law is where all of the three come together. It is modeled on the Kinetic Theory of Gases as well.


http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/idegas.html
Avagadro's Law
Avagadro's Law holds that temperature and pressure are constant, and the volume is directly proportional to the number of moles of gas present. Also, equal volumes of gases at the same temperature and pressure have the same number of particles.


http://www.chemteam.info/GasLaw/Gas-Avogadro.html
http://www.chemteam.info/GasLaw/Gas-Avogadro.html
Charles' Law
This law tells us that volume and temperature share a direct relationship at a constant temperature, the temperature being at Kelvin. To convert from Celsius to Kelvin, you simply add 273.15.


https://www.khanacademy.org/test-prep/mcat/physical-processes/gas-phase/v/charles-law
http://www.chemteam.info/GasLaw/Gas-Charles.html
https://www.khanacademy.org/test-prep/mcat/physical-processes/gas-phase/v/charles-law
http://www.chemteam.info/GasLaw/Gas-Charles.html
Boyle's Law
Boyle's Law holds that temperature and moles are constant. There are also conversions for pressure that you need to remember, such as pascals (101,325), torr (760), and a few more. Once you are able to make a data table of the numbers present, you simply plug into the formula below.

https://www.grc.nasa.gov/www/k-12/airplane/boyle.html
https://www.khanacademy.org/test-prep/mcat/physical-processes/gas-phase/v/boyles-law
https://www.grc.nasa.gov/www/k-12/airplane/boyle.html
https://www.khanacademy.org/test-prep/mcat/physical-processes/gas-phase/v/boyles-law
Intermolecular Forces
The below pictures show how to easily navigate intermolecular forces. By following the flowchart, you will be able to know what force is present. Fun fact: everything has SOME London Dispersion forces!!


http://chemistry.bd.psu.edu/jircitano/IMforces.html
https://www.khanacademy.org/science/organic-chemistry/gen-chem-review/electronegativity-polarity/v/intermolecular-forces-and-molecular-bonds
http://www.chem.ucla.edu/~harding/notes/notes_14C_noncoval02.pdf

http://chemistry.bd.psu.edu/jircitano/IMforces.html
https://www.khanacademy.org/science/organic-chemistry/gen-chem-review/electronegativity-polarity/v/intermolecular-forces-and-molecular-bonds
http://www.chem.ucla.edu/~harding/notes/notes_14C_noncoval02.pdf
Heating/Cooling Curves
Below are two examples of both a heating and cooling curve. As you can see, there are specific names for each phase change as a substance hits a certain temperature. You simply match up the amount of heat added with the temperature, and you are able to see the phase.


http://www.kentchemistry.com/links/Matter/HeatingCurve.htm
http://study.com/academy/lesson/what-are-heating-and-cooling-curves.html
https://www.youtube.com/watch?v=YG77v1PwQNM
http://www.kentchemistry.com/links/Matter/HeatingCurve.htm
http://study.com/academy/lesson/what-are-heating-and-cooling-curves.html
https://www.youtube.com/watch?v=YG77v1PwQNM
Monday, April 18, 2016
Biodiesel Facts
Here are some facts about biodiesel:
http://www.springboardbiodiesel.com/biobasics/bio-diesel-basics-that-everyone-should-know
http://www.soyatech.com/biodiesel_facts.htm
Below are cities that use biodiesel as well as cities that are beginning to:


http://www.springboardbiodiesel.com/biobasics/bio-diesel-basics-that-everyone-should-know
http://www.soyatech.com/biodiesel_facts.htm
Below are cities that use biodiesel as well as cities that are beginning to:

About Biodiesel
Biodiesel is the healthy, earth-friendly alternative to diesel gasoline. Made from natural products, it is more efficient as well as less harmful to the environment. In fact, it's remains can actually benefit the ecosystem where it is left!


http://biodiesel.org/
http://www.afdc.energy.gov/fuels/biodiesel.html
http://biodiesel.org/
http://www.afdc.energy.gov/fuels/biodiesel.html
Wednesday, April 6, 2016
Biodiesel Lab
In class, we made our own biodiesel! Below is a picture in the process:
We ended up using our biodiesel to propel our boats in the boat races. It paid off because my team took 2nd!
We ended up using our biodiesel to propel our boats in the boat races. It paid off because my team took 2nd!
Monday, April 4, 2016
Hybridization
Hybridization is the idea that atomic orbitals fuse to form newly hybridized orbitals, which in turn, influences molecular geometry and bonding properties. At first, it was a slightly challenging concept to grasp. But with practice, it became easy and I began to realize the patterns that never change.

https://www.khanacademy.org/science/chemistry/chemical-bonds/copy-of-covalent-bonds/v/sp3-hybrid-orbital-jay-final
https://chemistry.boisestate.edu/richardbanks/inorganic/bonding%20and%20hybridization/bonding_hybridization.htm
https://www.khanacademy.org/science/chemistry/chemical-bonds/copy-of-covalent-bonds/v/sp3-hybrid-orbital-jay-final
https://chemistry.boisestate.edu/richardbanks/inorganic/bonding%20and%20hybridization/bonding_hybridization.htm
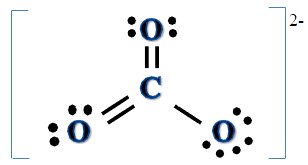
Resonance Structures
Resonance structures show the different ways in which bonding structures can be shown. In reality, all of the possible resonance structures are put together in the actual molecule. As shown in the picture below, this bonded molecule can be shown in these possible ways, even though it is essentially the same thing.
https://www.khanacademy.org/science/organic-chemistry/organic-structures/formal-charge-resonance/v/resonance-intro-jay
http://preparatorychemistry.com/Bishop_Resonance.htm

https://www.khanacademy.org/science/organic-chemistry/organic-structures/formal-charge-resonance/v/resonance-intro-jay
http://preparatorychemistry.com/Bishop_Resonance.htm
Electron Dot Formulas
Electron dot diagrams are visual ways of seeing how electrons are shared and distributed within bonds. They show the numbers of bonded pairs as well as lone pairs.
https://www.youtube.com/watch?v=y6QZRBIO0-o
https://www.khanacademy.org/science/chemistry/chemical-bonds/copy-of-dot-structures/v/drawing-dot-structures

https://www.youtube.com/watch?v=y6QZRBIO0-o
https://www.khanacademy.org/science/chemistry/chemical-bonds/copy-of-dot-structures/v/drawing-dot-structures

Sunday, March 6, 2016
Unit Exam
I am fairly confident that I did well on this exam. This unit came easier to me than others, and I put a lot of work into understanding the concepts. The biggest challenge was remembering all of the little details that are the difference between getting a question right and not getting it right, such as the conversions or exceptions. I took my time and made sure that I understood the questions, and I think that this paid off because Frank warned us that the biggest grade killer was reading the question wrong. Hopefully I did as well as I'm anticipating; my grade really needs it!!!!

Wavelength, Frequency, and Energy Calculations
This was the only math portion of this unit. Within it, there are 3 equations that helped us calculate wavelength, energy, and frequency. It took some time to get the hang of it because we had to remember things such as Planck's constant and multiplying by Avogadro's number when calculation moles/photon, but once it was practiced a lot, it became second nature. I studied this a lot for the quiz and got all of the problems right, and by the time the exam rolled around, I barely had to touch up on it to remember. This is probably one of my favorite math concept that we have done in this class!
http://www.chemteam.info/Electrons/calc-energy-freq-wavelength.html
http://www.kentchemistry.com/links/AtomicStructure/waveequations.htm

http://www.chemteam.info/Electrons/calc-energy-freq-wavelength.html
http://www.kentchemistry.com/links/AtomicStructure/waveequations.htm
Electron Configuration
This topic was probably the easiest to grasp for me. You just have to remember the four subshells and their locations (Smart People Drop First semester), and then you are able to formulate a configuration for an element. It is essentially their address, and it leads you to where they are located. It was fairly easy to make a configuration for an element as well as locate an element from a given configuration. There are 6 exceptions that we were accountable for, and they weren't difficult to remember either.


Periodic Trends
This lesson was one that took extra work in order to memorize and utilize accurately. Since there are several, remembering which way the trends go on the periodic table for all is crucial. I'll admit that I did not take the time to figure out what each means necessarily, I just made sure I remembered the direction of each.
http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
https://www.khanacademy.org/science/chemistry/periodic-table/periodic-table-trends-bonding

http://chemwiki.ucdavis.edu/Core/Inorganic_Chemistry/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends
https://www.khanacademy.org/science/chemistry/periodic-table/periodic-table-trends-bonding

Quantum Numbers
Quantum numbers are a fairly easy concept to get. There isn't application, but it is simply memorization of what the four numbers mean and knowing how to locate elements from a set of numbers.
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch6/quantum.html
https://www.khanacademy.org/science/chemistry/electronic-structure-of-atoms/orbitals-and-electrons/v/quantum-numbers
These are two sources that I found helpful in putting this concept into perspective.

http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch6/quantum.html
https://www.khanacademy.org/science/chemistry/electronic-structure-of-atoms/orbitals-and-electrons/v/quantum-numbers
These are two sources that I found helpful in putting this concept into perspective.

Thursday, February 18, 2016
Flame Test Lab
We did the flame test lab today in class. I think this was one of the cooler labs we have done, and it was really interesting to see how different metals burn in different colors.
I'm not sure what those rings are, but it looks awesome... :P
This is the source we used to find the wavelengths from knowing the colors: https://sites.google.com/site/thecreativechemistsprogram/flame-test-lab
Tuesday, February 16, 2016
Review of Acids and Bases
http://www.chemtutor.com/acid.htm
This was a website that I found very valuable in reviewing acids and bases. It provides everything you need to know from the properties of acids and bases to how to conduct titration. I wish that I would have had this source in studying for the test.


This was a website that I found very valuable in reviewing acids and bases. It provides everything you need to know from the properties of acids and bases to how to conduct titration. I wish that I would have had this source in studying for the test.
Titration
Titration was the most challenging aspect of this unit for me. Although the problems are generally conducted in the same way, it becomes confusing whenever there are contradicting mole ratios and confusingly worded questions. I would say that this was definitely the thing I struggled with most during the exam.
Here is an intro to titration: https://www.khanacademy.org/science/chemistry/acid-base-equilibrium/titrations/v/titration-introduction

Here is an intro to titration: https://www.khanacademy.org/science/chemistry/acid-base-equilibrium/titrations/v/titration-introduction
Post Exam Thoughts
Before taking the exam, I felt like I had a pretty good idea as to what I was doing with acids and bases and titration... After, not so much. The way that the questions were worded on this exam really threw me off, so I had to definitely make educated guesses on a few. I hope that what I knew led me to get a decent grade, we'll see!!
http://www.chem4kids.com/files/react_acidbase.html
http://www.chem4kids.com/files/react_acidbase.html
Friday, January 29, 2016
Acid/Base Strength
Acid and base strength depend on different things.
Acid strength depends on the list of strong acids and also whether the oxygens outnumber the hydrogens by more than two. The chart below shows the memorized list of strong bases:

Strong bases only depend on one thing: whether they have a group 1 or group 2 metal in them.

Here is a quiz to review: http://chemistry.about.com/od/acidsbase1/l/blacidbases.htm
Acid strength depends on the list of strong acids and also whether the oxygens outnumber the hydrogens by more than two. The chart below shows the memorized list of strong bases:
Strong bases only depend on one thing: whether they have a group 1 or group 2 metal in them.
Here is a quiz to review: http://chemistry.about.com/od/acidsbase1/l/blacidbases.htm
Conjugate Acids & Bases
Conjugate acids and bases are found by using Bronsted-Lowery. In this method, acids donate a proton (H+) and bases accept a proton. Basically, the acid on the left side of the equation donates a proton to the product, making it the conjugate base. The base on the left side accepts a proton, making its product the conjugate acid.


Above are two ways of showing conjugate acids and bases.
https://www.khanacademy.org/science/chemistry/acids-and-bases-topic/copy-of-acid-base-equilibria/v/conjugate-acids-and-bases
http://chemwiki.ucdavis.edu/Physical_Chemistry/Acids_and_Bases/Acid%2F%2FBase_Reactions/Conjugate_Acids-base_Pairs

Above are two ways of showing conjugate acids and bases.
https://www.khanacademy.org/science/chemistry/acids-and-bases-topic/copy-of-acid-base-equilibria/v/conjugate-acids-and-bases
http://chemwiki.ucdavis.edu/Physical_Chemistry/Acids_and_Bases/Acid%2F%2FBase_Reactions/Conjugate_Acids-base_Pairs
Monday, January 25, 2016
Vitamin C Lab
Today, we started the Vitamin C lab. It is essentially testing different common drinks, such as apple juice and V8, for their vitamin C concentration. You add 20 drops of the liquid along with 3 drops of starch, and then you continue to add iodine drops until the solution remains a dark blue color. It was really interesting to see how different drinks that people drink every day have such different concentrations of this citrus-y vitamin even though they're all fruity.

Here is a link that may help with this unit: http://hyperphysics.phy-astr.gsu.edu/hbase/hph.html

Here is a link that may help with this unit: http://hyperphysics.phy-astr.gsu.edu/hbase/hph.html
Thursday, January 21, 2016
Unit Test
Tomorrow, we are taking the unit exam for aqueous solutions. Since I didn't do as well as I wanted to on the quiz, I need to study extra tonight, so I have included good sources that could help with refreshing and studying the below topics.
We have learned about dilutions, types of solution formations, solubility lines, and how to use molarity in stoich calculations.
http://www.chemteam.info/Solutions/Molarity.html
http://www.occc.edu/kmbailey/Chem1115Tutorials/Molarity.htm
https://www.youtube.com/watch?v=SXf9rDnVFao
https://www.youtube.com/watch?v=rPND65LPwS0
http://chemcollective.org/activities/tutorials/stoich/solution_stoi
We have learned about dilutions, types of solution formations, solubility lines, and how to use molarity in stoich calculations.
http://www.chemteam.info/Solutions/Molarity.html
http://www.occc.edu/kmbailey/Chem1115Tutorials/Molarity.htm
https://www.youtube.com/watch?v=SXf9rDnVFao
https://www.youtube.com/watch?v=rPND65LPwS0
http://chemcollective.org/activities/tutorials/stoich/solution_stoi
Thursday, January 14, 2016
Molarity
Molarity is one of the basic principles of this unit. The formula is depicted bellow. Another way to think of molarity is by saying the concentration of a solution because it is measured by the amount of moles per every one liter, meaning it is the concentration of it.
Also, here are a few examples of running molarity problems in all three ways possible:

Murder Lab
We finished up the murder lab today in class by weighing our final product and filter paper. We concluded that the murder weapon was AgNO3, and the molarity was around 0.18M. According to these two facts, and by looking at all of the suspect information, it was found that Mr. Green killed Miss Scarlett.
Below is a picture of our experiment before the solid was fully filtered.
Below is a picture of our experiment before the solid was fully filtered.

Friday, January 8, 2016
Good to the Last Drop Lab
Our first lab of this unit was simple and to the point. After learning dilutions today, we got to test it out ourself with water and food coloring. Below is the procedure:
We then figured out solution B to be 0.2D and solution C to be 0.02D.
Subscribe to:
Comments (Atom)






